Background
Timeline
- May 2020 – Concept and Design
- August 2020 - Patent filing
- September 2020 - Mutiple Revisions to the Model
- October 2020 - Prototype
- November 2020 - Design/Improvements
- March 2021 - Production
- April 2021 - Shipments to begin
Our mask is made with both professional and regular daily use in mind. We have designed a product that is comfortable enough to wear every day, all day, and easily sterilized for reuse.
We currently have two models available, Base Model, and Model 360.
** Patent Pending Technology **
Base Model
Our base model, the clear tilium N95 mask is stylish and made with breathable and comfortable material so the person wearing can communicate with confidence and visual expressions. This mask is for daily use and could be reused and sanitized. Most importantly, available in multiple sizes to fit all.

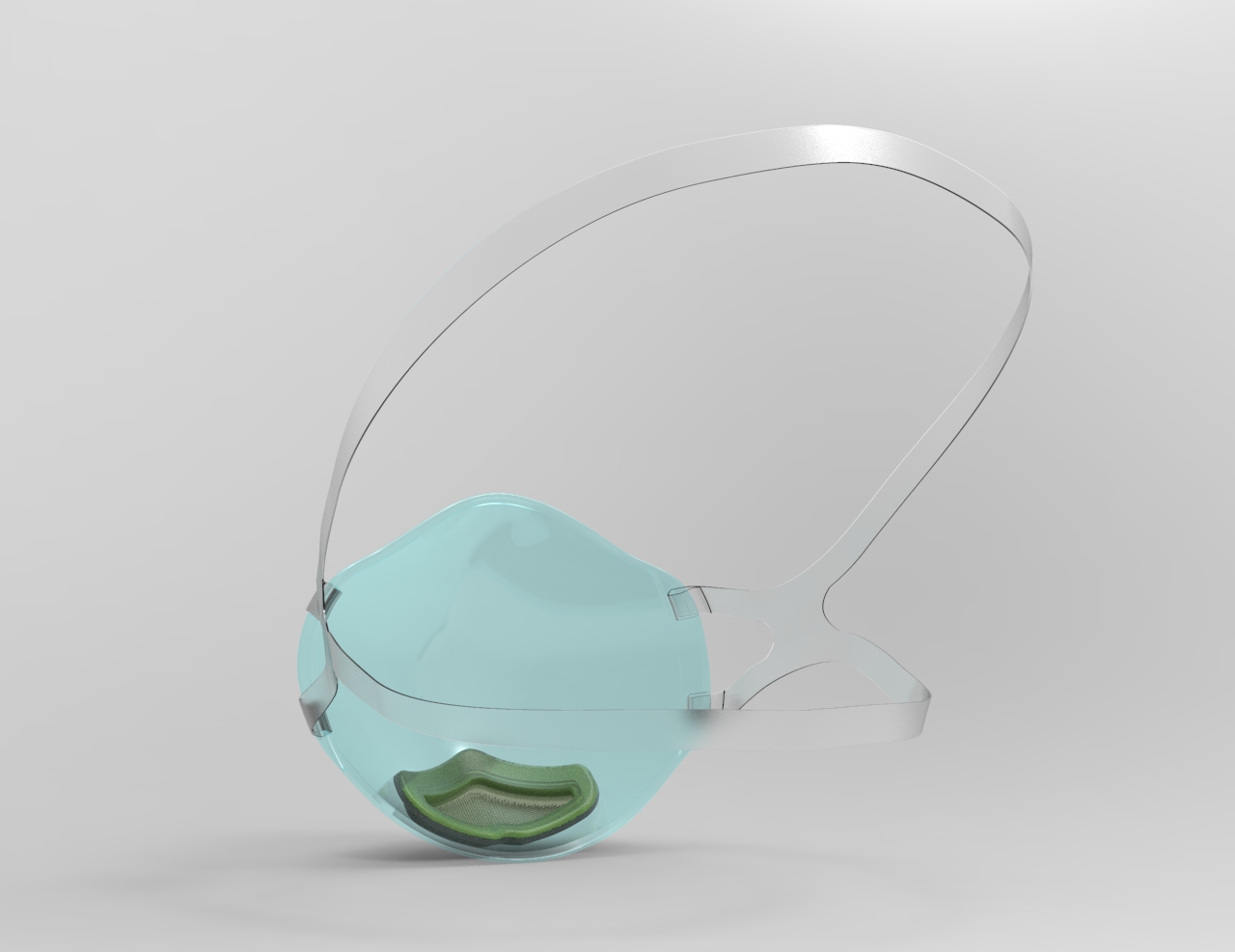
- Cutting edge technology; activated carbon, aerospace grade hepa, UVC power technology filter
- Transparency; improves self-esteem and social communications
- Facial Recognition; enables you to utilize facial recognition software
- Five Layer Filter Protection; remove 95% of .25 + micron-particles
- Superior Protection; protect your mouth from dust, germs, allergens, smoke, pollution etc.
- Easily Replaceable filters
- Price: $70 (includes 5 replaceable filters)
Model 360
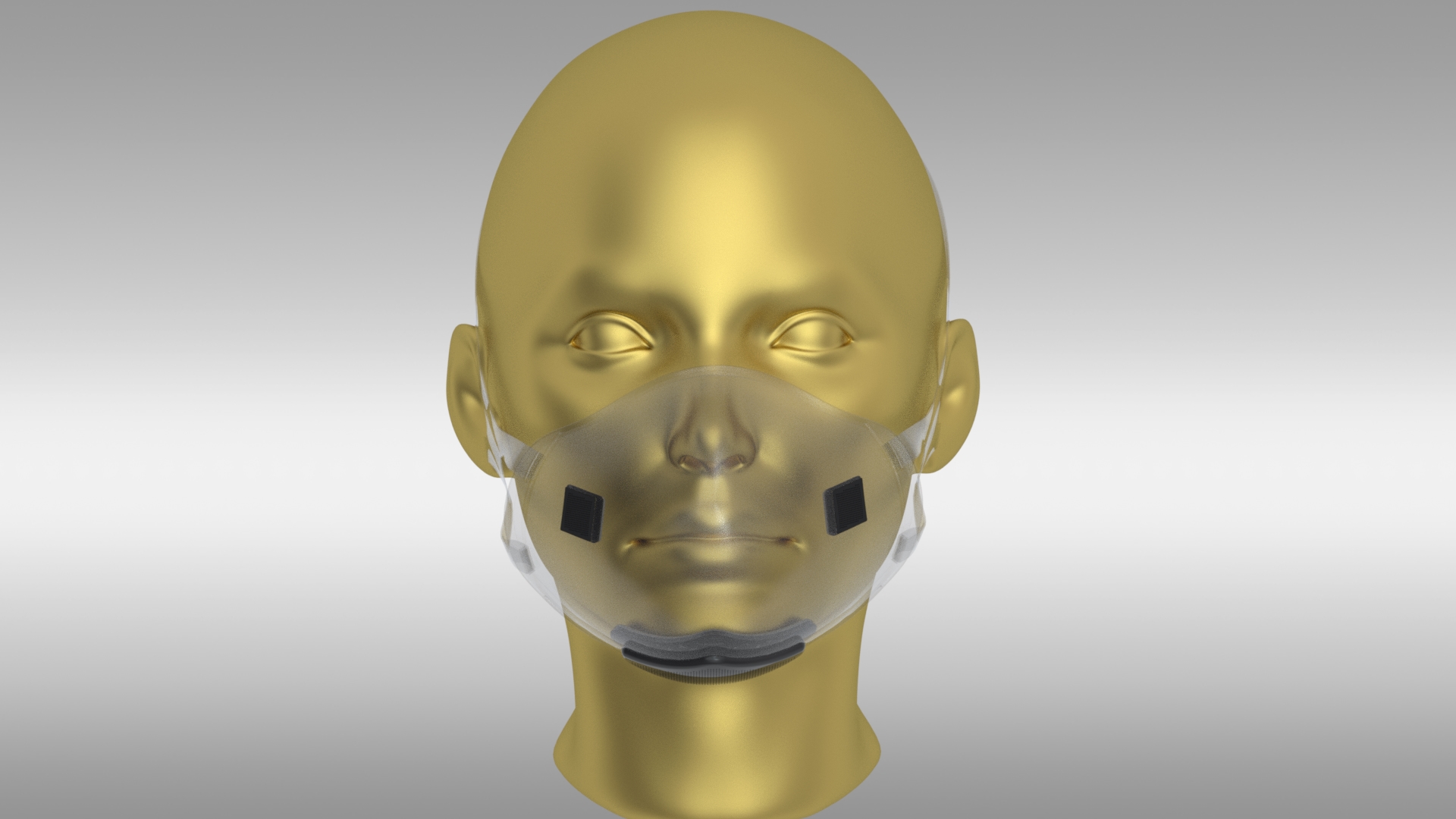
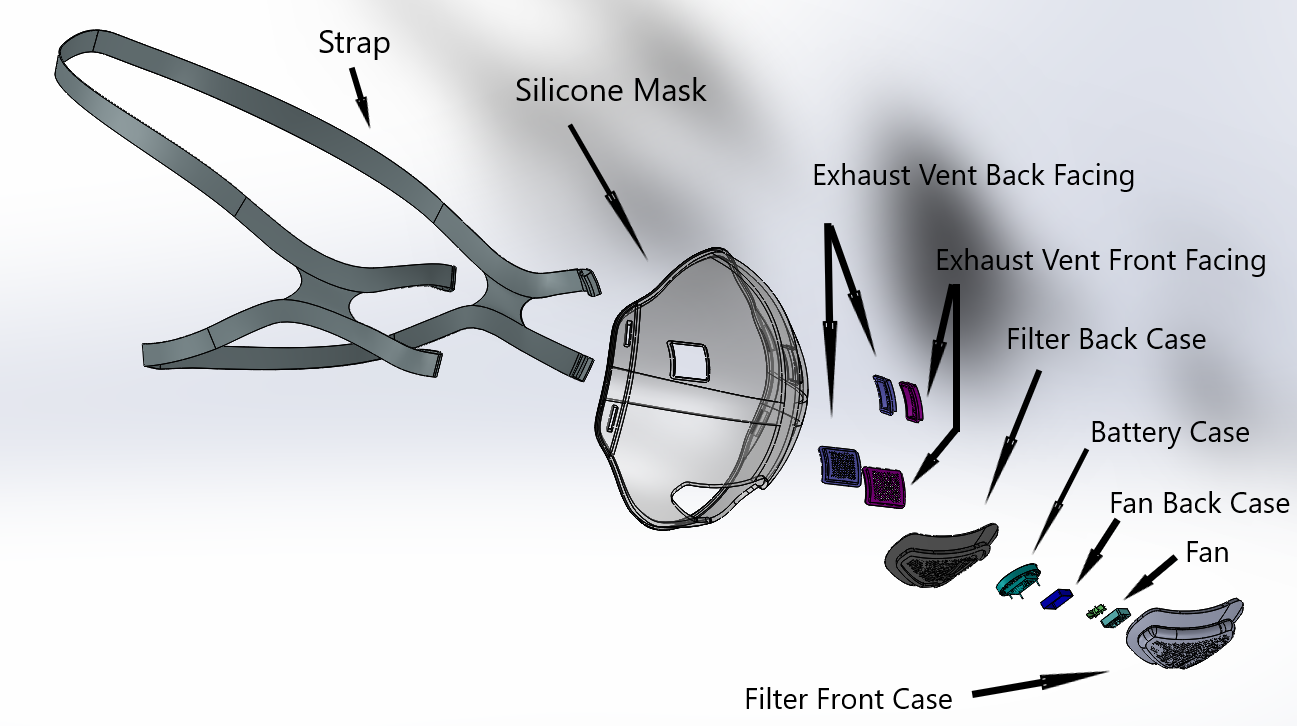
- Cutting edge technology; activated carbon, aerospace grade hepa, UVC power technology filter
- Transparency; improves self-esteem and social communications
- Facial Recognition; enables you to utilize facial recognition software
- Five Layer Filter Protection; remove 95% of .25 + micron-particles
- Superior Protection; protect your mouth from dust, germs, allergens, smoke, pollution etc.
- Easy airflow with microfan
- Battery life is expected to be about 10 hours with each full charge
- Easily Replaceable filters and battery
- Price: $135 (includes 5 replaceable filters)
- Recharging pack: $65 (one charger, 2 rechargeable batteries)
- Battery pack: $40 (2 rechargeable batteries)
N-95 Respirators
There are various types of masks out there. During the COVID19 outbreak, N95 masks, once labeled as strictly professional, have quickly become common. N95 masks are a lot more reliable compared to the typical surgical masks or handmade face masks. Compared to the regular surgical masks, specialized N-95 respirators are tight-fitting, reliable, and aren’t prone to leakage. Therefore, if approached wisely, N-95 filters work very well when it comes to protecting people from the new deadly disease.
The similarities among surgical masks and surgical N-95s are:
- They are tested for fluid resistance, filtration efficiency (particulate filtration efficiency and bacterial filtration efficiency), flammability and biocompatibility.
- They should not be shared or reused.
N-95 respirators are examples of personal protective equipment that are used to protect the wearer from airborne particles and from liquid contaminating the face. The Centers for Disease Control and Prevention (CDC) National Institute for Occupational Safety and Health (NIOSH) and the Occupational Safety and Health Administration (OSHA) also regulate N-95 respirators.
It is important to recognize that the optimal way to prevent airborne transmission is to use a combination of interventions from across the hierarchy of controls, not just Personal Protective Equipment (PPE) alone.
N
95
.25 micron
Material
N-95 General Respirator Precautions
- People with chronic respiratory, cardiac, or other medical conditions that make breathing difficult should check with their health care provider before using an N-95 respirator because the N-95 respirator can make it more difficult for the wearer to breathe.
- Replace filter frequently for best results.
- If your respirator is damaged or soiled, or if breathing becomes difficult, you should remove the respirator, discard it properly, and replace it with a new one. To safely discard your N-95 respirator, place it in a plastic bag and put it in the trash. Wash your hands after handling the used respirator.
- N-95 respirators are not designed for children or people with facial hair. Because a proper fit cannot be achieved on children and people with facial hair, the N-95 respirator may not provide full protection.
N-95 How to Wear an N95 Mask Properly?
- Sanitize your hands and put on your N95 respirator mask.
- Ensure that your clean N95 mask doesn't have any defects such as tears. Ensure, the filters are clean (replace filters as required)
- Hold the N-95 respirator face mask in your palm with the mouthpiece at fingertips, while hanging the head straps loosely.
- Position your reusable N-95 mask below your chin with the mouthpiece up.
- While holding the N-95 mask in place, pull the top strap behind your head and leave it to rest high on the back of your head.
- Position the bottom strap around the back of your neck, below your ears.
- Untwist the straps of your washable N-95 mask.
- Position both your palms over the respirator to check if it fits your face right.
- After you put on the mask, don’t forget to sanitize your hands again.
N-95 Respirators in Industrial and Health Care Settings
Most N-95 respirators are manufactured for use in construction and other industrial type jobs that expose workers to dust and small particles. They are regulated by the National Personal Protective Technology Laboratory (NPPTL) in the National Institute for Occupational Safety and Health (NIOSH), which is part of the Centers for Disease Control and Prevention (CDC).
However, some N-95 respirators are intended for use in a health care setting. Specifically, single-use, disposable respiratory protective devices used and worn by health care personnel during procedures to protect both the patient and health care personnel from the transfer of microorganisms, body fluids, and particulate material. These surgical N-95 respirators are class II devices regulated by the FDA, under 21 CFR 878.4040, and CDC NIOSH under 42 CFR Part 84.
N-95s respirators regulated under product code MSH are class II medical devices exempt from 510(k) premarket notification, unless:
- The respirator is intended to prevent specific diseases or infections, or
- The respirator is labeled or otherwise represented as filtering surgical smoke or plumes, filtering specific amounts of viruses or bacteria, reducing the amount of and/or killing viruses, bacteria, or fungi, or affecting allergenicity, or
- The respirator contains coating technologies unrelated to filtration (e.g., to reduce and or kill microorganisms).


 fb.com/synthiumhealth
fb.com/synthiumhealth /synthiumhealth
/synthiumhealth