Principle Of The Test
The test uses the principle of colloidal gold immunochromatography and capture method to detect COVID-19 (SARS-CoV-2) IgM and IgG antibodies in human serum, plasma, and whole blood. When the sample contains COVID-19 IgM / IgG antibody and the concentration is greater than or equal to the minimum detection limit, the antibody binds to the antigen immobilized on colloidal gold nanoparticles which migrate to test region 1 (T1) / test region 2 (T2), where it is captured by the secondary antibody to produce a red reaction line. The result is considered positive when a red reaction line appears in either test region. The result is considered negative when no red reaction line is in test line region. The test is valid when the control line (C) produces a reaction line and invalid if no control line (C) appears.
Key Features and Benefits
- Works with venous whole blood, serum or plasma and finger prick whole blood
- A rapid test that takes at most 15 minutes from sample collection to results interpretation
- Precise test results ready in 10 minutes
- Aid in the screening and diagnosis of COVID-19 virus, in combination with PCR
- Room temperature shipping and storage
- No instrument required
- Aid in risk stratification (potential to be used in doctor’s office, ER, airports, schools, etc.): non-immune (IgG/IgM), early infection (IgM only), late infection (IgM/IgG), immune (IgG only)
Simple Procedure
- Collect serum/plasma/blood sample
- Mix serum/plasma sample with diluent and add to sample well (venous) or add blood sample and diluent to sample well (finger prick)
- Precise test results ready in 10 minutes
- Read results at 10 minutes
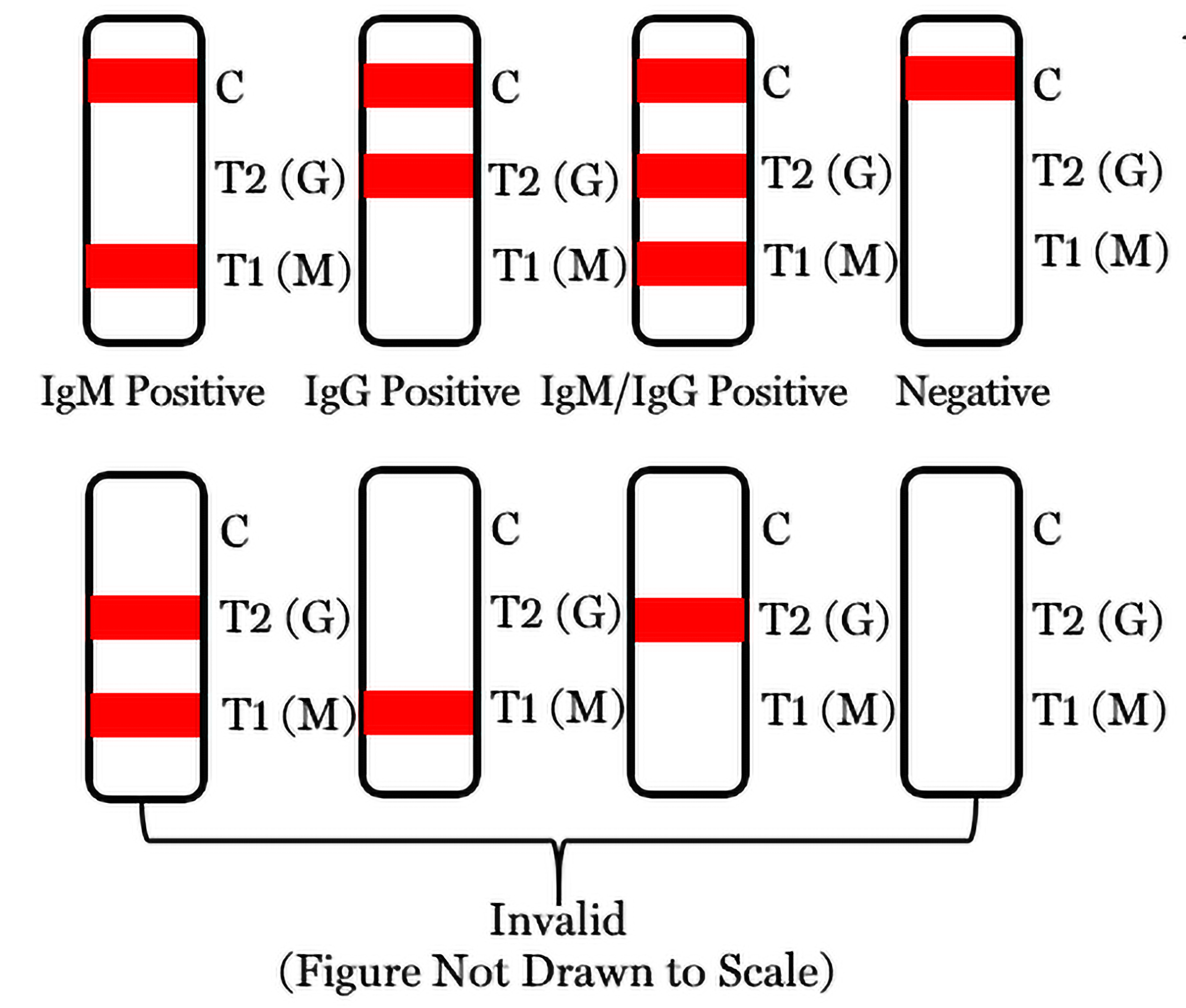
Results Interpretation
- Positive for SARS-CoV-2 IgM: A red line appears in test region 1 (T1)/M as well as the control line (C).
- Positive for SARS-CoV-2 IgG: A red line appears in the test region 2 (T2)/G as well as the control line (C).
- Positive for Both SARS-CoV-2 IgM and IgG: A red line appears in both test region 1 (T1)/M and test region 2 (T2)/G as well as the control line (C).
- Negative for SARS-CoV-2 IgM/IgG: A red line appears in the control line (C) but no line appears in test region 1 (T1)/M or test region 2 (T2)/G.
- Invalid Result: test is invalid if no red line appears in the control line (C).
Nirmidas COVID-19 Test Kit
Test:
COVID-19 IgG/IgM Rapid Test
Specimen:
Whole Blood/Serum/Plasma
Form:
Cassette
Packaging:
10 tests/box
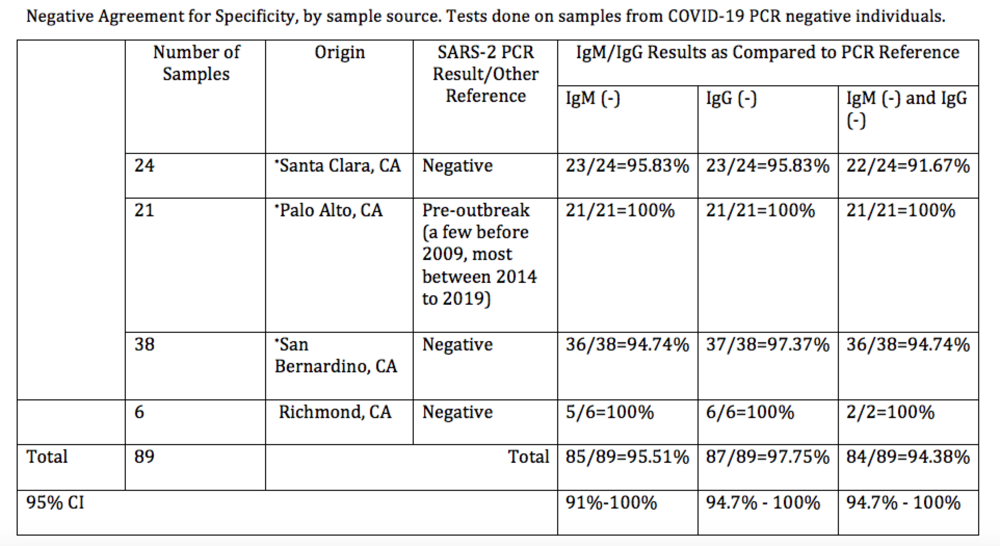
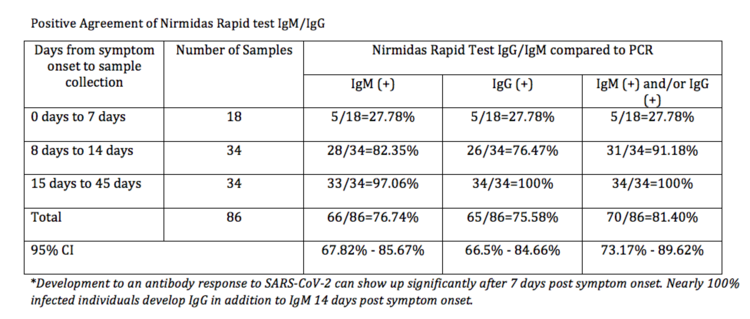
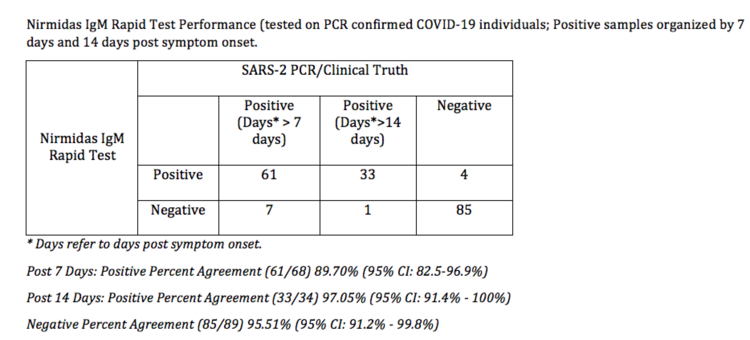
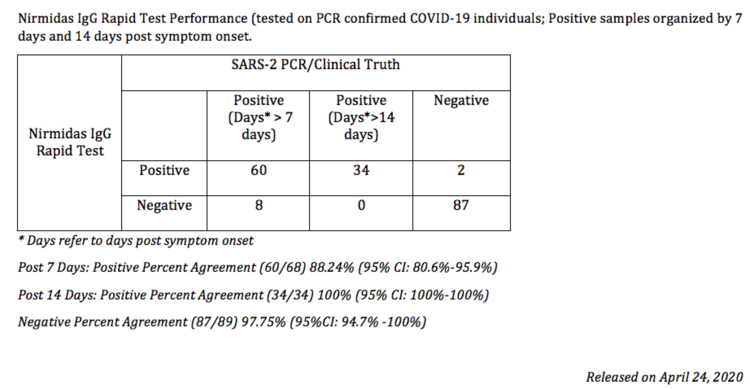
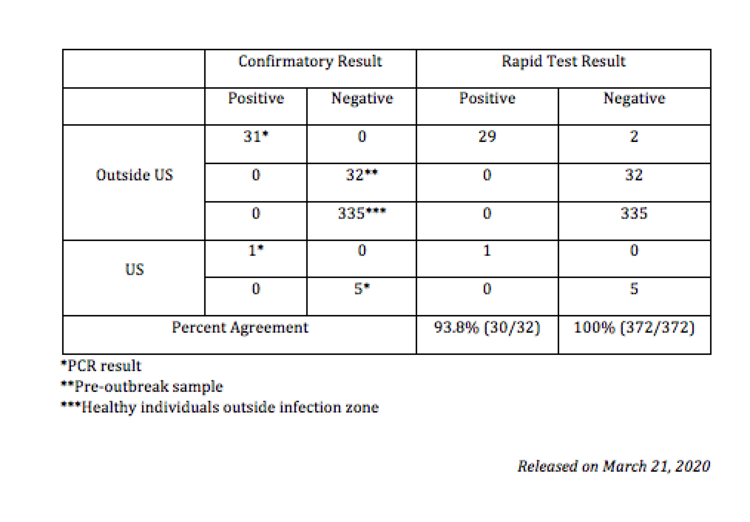
$250 ($25/test kit) | PURCHASEPerformance Summary Data
CAPILLARY BLOOD SAMPLE TUTORIAL
Precautions
- For professional in vitro diagnostic use only. Do not use after expiration date;
- Do not eat, drink or smoke in the area where the specimens or kits are handled;
- Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout testing and follow the standard procedures for proper disposal of specimens;
- Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are being tested;
- Humidity and temperature can adversely affect results.
FDA Notification
Nirmidas has notified the FDA that we have validated and are offering our serology test, Nirmidas COVID-19 (SARS-CoV-2) IgM/IgG Antibody Detection Kit, to commercial laboratory and for POCT settings, in compliance with Section IV.D. of the FDA’s Policy for Diagnostic Tests for Coronavirus Disease-2019 . The FDA has not reviewed the Nirmidas’ validation of this test. Nirmidas will be pursuing EUA of this test.
If you have any queries regarding the product, please contact us.


 fb.com/synthiumhealth
fb.com/synthiumhealth /synthiumhealth
/synthiumhealth