Overview
The BD Veritor™ System for Rapid Detection of SARS-CoV-2 is a chromatographic immunoassay for the direct and qualitative detection of SARS-CoV-2 antigens in nasal swabs from patients with signs and symptoms who are suspected of COVID-19.
Key Features and Benefits
Simplify the testing process
- Easy operation and 1-button functionality may help reduce manual test processing errors
- Simple sample processing with color-coded tubes
- Displays easy-to-read digital results for COVID-19 in 15 minutes: “CoV2: +” for positive, “CoV2: -” for negative
- Records results on secured internal drive
- Advanced particle technology helps improve test performance*
- Adapts easily to your workflow by offering 2 operational modes
- Walk away: Test device is inserted immediately into Analyzer, so staff can multi-task while sample incubates (15 minutes)
- Analyze now: Test device is inserted after incubation time is complete, allowing batches of samples to be tested (results in seconds)
- Download and display Lot number, patient/specimen ID, Operator ID and test records with BD Veritor™ InfoScan module
- Offers result-printing capabilities via USB port
Easy sample preparation workflow
These are the steps for sample preparation, including the process for each workflow mode of the BD Veritor™ Plus System.




Results Interpretation
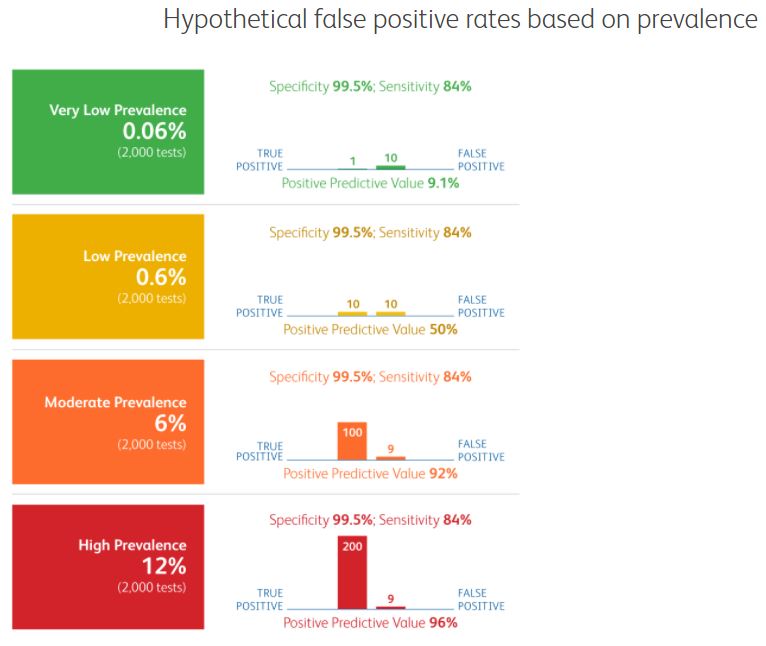
It is important to understand how local disease prevalence rate and test performance characteristics interact to influence the proportion of false positive test results compared to all positive results. The infographic below demonstrates how very low, low, medium and high prevalence rates make a difference. It is important to note that your institution’s specific prevalence rates can be used to calculate the potential in your facility. Understanding that false positive test results are a possibility is important. Please, follow your institution or state and local guidance for both addressing a patient with a positive test result. Guidance may include precautionary isolation procedures and a second mode of testing for confirmation.
BD 256082 Veritor SARS-CoV-2 Rapid Detection Kit - 30/box *BACKORDER*
Test:
COVID-19 Antigen Rapid Test
(BD Veritor™ Plus Analyzer should be purchased separately)
Specimen:
Nasal Swab
What's in the Box?
30x single use test devices
30x single use reaction tubes, each with 325 µL extraction reagent and an integral dispensing tip
30x sterile, single use specimen sampling swabs
1x SARS-CoV-2 (+) control swab for single use
1x SARS-CoV-2 (-) control swab for single use
Instructions for use
Packaging:
30 tests/box
Non-refundable
$1,125 ($37.50/test kit) | PURCHASEProduct Brochure
BD Veritor(tm) System for Rapid Detection of SARS-CoV-2 with the BD Veritor(tm) Plus Analyzer
Precautions
- For professional in vitro diagnostic use only. Do not use after expiration date;
- Do not eat, drink or smoke in the area where the specimens or kits are handled;
- Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout testing and follow the standard procedures for proper disposal of specimens;
- Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are being tested;
- Humidity and temperature can adversely affect results.
FDA Notification
- This test is not FDA cleared or approved.
- It has been authorized by the FDA under an Emergency Use Authorization for use by authorized laboratories.
- It has been authorized only for the detection of proteins from SARS-CoV-2 and not for any other viruses or pathogens.
- This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for the detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
If you have any queries regarding the product, please contact us.


 fb.com/synthiumhealth
fb.com/synthiumhealth /synthiumhealth
/synthiumhealth